Regarding the Partnership Agreement for Clinical Trials between A2 and NRGJ, a subsidiary of the National Cancer Institute in the United States
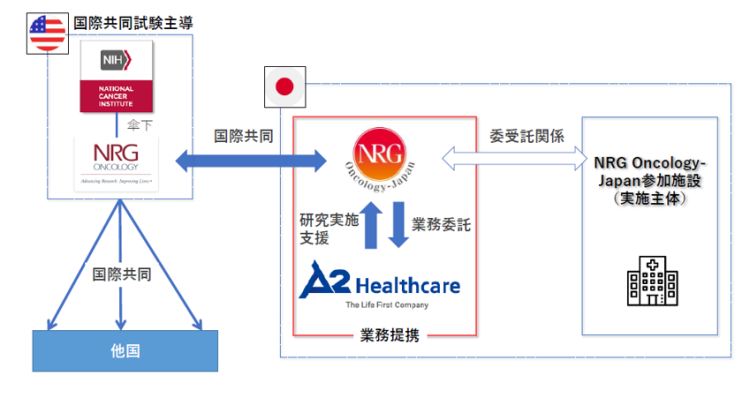
A2 Healthcare Corporation (Headquarters: Bunkyo-ku, Tokyo; President: Hitoshi Kamiya; hereinafter “A2”) and NRG Oncology-Japan(hereinafter ”NRGJ”), a subsidiary of the National Cancer Institute (“NCI”) in the United States and a multi-facility joint trial promotion entity under NCI in Japan, have entered into a Partnership Agreement for Clinical Trial. With this partnership, A2 aims to advance its support services for clinical trials involving unapproved drugs in Japan, addressing the pressing societal issues of “drug lag” and “drug loss”. Through this collaboration, A2 contributes to the resolution of these challenges and promote clinical trial support services in the field of unapproved drugs in the country.
Since the 1970s, NCI has been a government agency involved in the development of many anticancer drugs in the United States. NRGJ, as an international joint research lead organization under NCI, is conducting multi-center joint clinical trials for many types of cancer in Japan. Meanwhile, A2 has established an Oncology Special Division in 2010, undertaking a significant role in conducting corporate-driven and physician-initiated clinical trials for anti-cancer drug development in Japan. With approximately one hundred oncology trials for clinical monitoring across a wide range of cancer types, A2 has been as a top-tier domestic CRO, boasting a wealth of expertise and support in the field of cancer research and development.
With the execution of this partnership agreement, A2 will conduct a greater number of oncology trials and generate more evidence for unapproved drugs in Japan led by NRGJ. This is expected to contribute to the improvement of the quality of cancer care in Japan by delivering necessary treatments and medications to domestic cancer patients more promptly.
Through these collective endeavors, A2 is committed to addressing the societal challenges of drug lag and drug loss, contributing to a society where better healthcare is provided. We aim to make a positive impact and contribute to the advancement of medical services.
※Source: Annual Trends in the Number and Percentage of Unapproved Drugs in Japan
(Excerpted from the Japan Pharmaceutical Manufacturers Association website)
https://www.jpma.or.jp/news_room/newsletter/205/05pc-01.html
 ーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーー
ーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーーー
General Incorporated Association NRG Oncology-Japan
Office Minato-ku, Tokyo
Established 2015
Business content As a global member of “NRG Oncology”, a multi-center joint study group under the umbrella of the
National CancerInstitute (“NCI”) in the United States, NRG Oncology-Japan is focusing on
gynecologicalcancer, breast cancer,and radiotherapy, and have expanded to include gastrointestinal,
respiratory, urinary, and head and neck areas, and conducted NRG Oncology studies in Japan
For more infomation:
Regarding-the-Partnership-Agreement-for-Clinical-Trials-between-A2-and-NRGJ-a-subsidiary-of-the-National-Cancer-Institute-in-the-United-States
Contact: Corporate Planning Section, Corporate Planning Department marke@a2healthcare.com
