Joint Research and Development Agreement on “Human (Allogeneic) Subcutaneous Adipose Tissue-Derived Mesenchymal Stem Cell Sheet (PAL-222) for Patients with Myopic Chorioretinal Atrophy
PharmaBio Corporation (Headquarter: Nagoya, Aichi; President and CEO: Hitoshi Kusano; hereinafter “PharmaBio”) and A2 Healthcare Corporation (Headquarter: Bunkyo-ku, Tokyo; President and CEO: Hitoshi Kamiya; hereinafter “A2”) have entered into a joint research and development agreement dated July 14, 2023, on “human (allogeneic) subcutaneous adipose tissue-derived mesenchymal stem cell sheet (PAL-222 (*1)) for the treatment of myopic chorioretinal atrophy (hereinafter “this disease”)”.
With regards to this disease, no effective treatment has yet been developed, and patients eventually suffer from social blindness (*2). PharmaBio has developed a regenerative medicine product (PAL-222) by using its proprietary manufacturing technology for cell sheet which consists solely of extracellular matrix components produced by the cells themselves, and has conducted research on treatment for this disease. PAL-222 is currently in the clinical trial phase (Phase I/IIa clinical trial (PAMyCA study)).
A2 has been seeking a new method of clinical development support beside conventional clinical development support services. By utilizing the extensive experience in the development of regenerative medicine products in Japan and overseas as “Pipeline Accelerator”, A2 has concluded that PAL-222 is a product with high development value that will enable us to solve unmet medical needs through innovation. In addition, we expect that the product technology of PAL-222 will play an essential role in realizing innovative, unprecedented treatment methods not only in Japan but also overseas.
PharmaBio and A2 signed a “Business Alliance Agreement” in May 2016. Through the conclusion of this joint research and development agreement, the two companies will make every effort to deliver the medical product to patients who are waiting for treatment of this disease as soon as possible.
(*1) About PAL-222
PAL-222 is a mesenchymal stem cell sheet with an endogenous scaffold structure. The cell sheet is fabricated by the world’s first cell sheet creation method with Bruch’s membrane-like structure composed only of extracellular matrix components produced by the cells themselves, a proprietary technology of Professor Tsutomu Yasukawa of Nagoya City University Graduate School of Medical Sciences, and is developed by PharmaBio. Compared to transplantation of cell suspensions, the new sheet has the potential to: (1) improve cell engraftment rate, (2) exhibit functions by transplanted cells (leading to improving and maintaining of visual function), (3) reduce the risk of complications and be less likely to cause allogeneic rejection reaction owing to the characteristics of mesenchymal stem cells. They are also expected to have a protective effect on the surrounding tissue functions by secreting various growth hormones and cytokines.
A Phase I/IIa clinical trial (PAMyCA study) has started this year at Nagoya City University Hospital( https://www.pharmabio.co.jp/news/view/43).
(*2) Blindness at a level that interferes with daily life (corrected visual acuity of 0.02 or less in Japan)
【Figure: Positioning of PAL-222 in the treatment of pathologic myopia】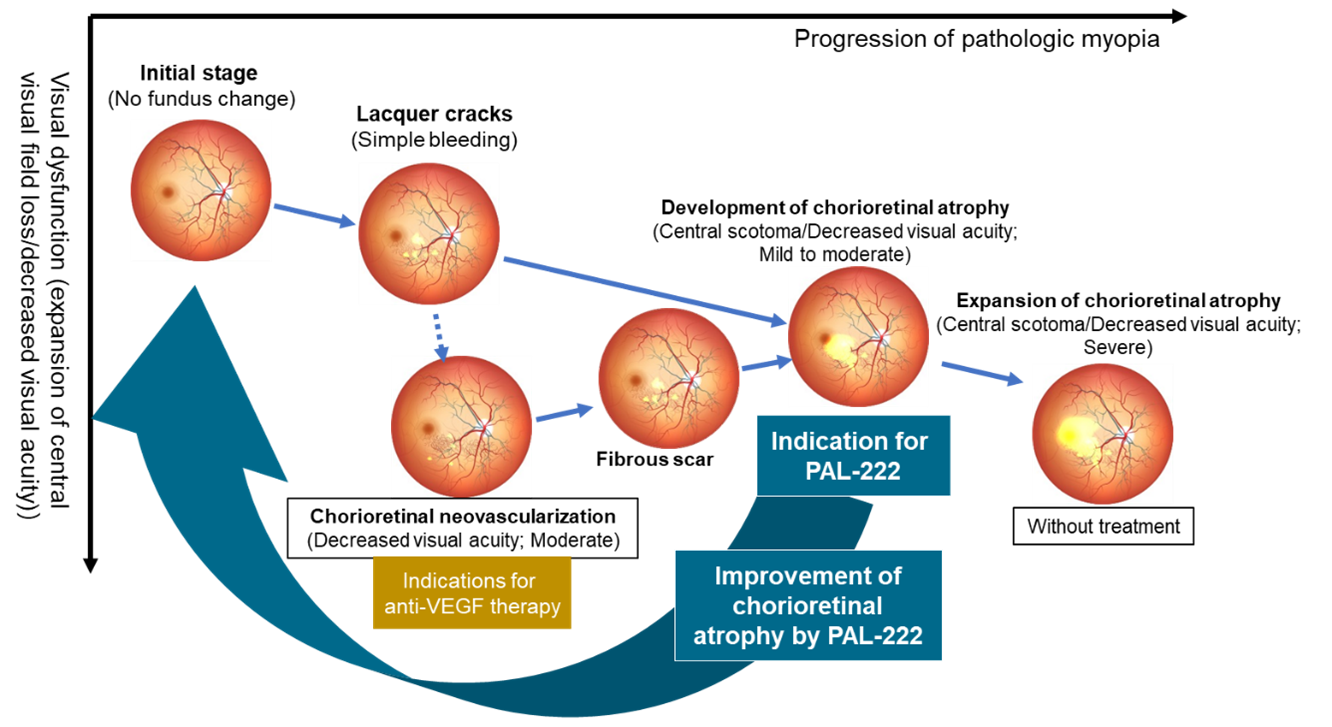
【About PharmaBio Corporation】
Ever since established in October 2010, PharmaBio’s main business is development of regenerative medicine products, development of manufacturing processes and contract manufacturing of cellular pharmaceuticals, and contract testing and inspection services such as cellular microbiological safety testing. PharmaBio started contract manufacturing business of regenerative medicine products ahead of other companies in 2011 and have been providing GMP/GCTP compliant services to global companies and others. Based on its solid manufacturing knowledge, PharmaBio is engaged in the development of new regenerative medicine products as well as high quality contract manufacturing services.
PharmaBio Corporation:https://www.pharmabio.co.jp/en/index.html
【About A2 Healthcare Corporation】
A2 is one of the industry’s leading CROs (Contract Research Organizations), conducting clinical trials under contract from pharmaceutical companies, and has been involved in the development of numerous drugs with approximately 1,100 employees in Tokyo and Osaka. In addition to the traditional lifestyle-related disease area, A2 in the development of drugs related to cancer, central nervous system, respiratory disease, vaccine, and also promotes the introduction of various advanced solutions such as RBM, eSource Data, eSubmission, and DCT(Decentralized Clinical Trials) to improve the efficiency of clinical trials. A2Healthcare is also a subsidiary of ITOCHU Corporation and forms the core of the group’s healthcare business.
A2 Healthcare Corporation: http://www.a2healthcare.com
【Please contact below for any question】
PharmaBio Corporation
Project Promotion Department
E-mail:ir_admin@pharmabio.co.jp
A2 Healthcare Corporation
Business Development Division
Frontier Business Department
E-mail:marke@a2healthcare.com
